Electronegativity and Bonding
A covalent bond has a shared pair of electrons. In $H_{2}$, the bonded atoms are identical, with each atom having an equal share of the electrons. $H-H$ is non-polar, due to this equal distribution of charge.
When the bonded atoms are different, one of the atoms is more likely to attract the electrons. The bonded atom with the greater attraction for the electrons is said to be more electronegative.
Electronegativity
Electronegativity is a measure of the attraction of a bonded atom for the pair of electrons in a covalent bond.
In a $HCl$ molecule, the $Cl$ atom is more electronegative meaning it has a greater attraction to the bonded electron pair. This causes a permanent dipole.
Permanent Dipole
A permanent dipole is a small charge difference across a bond due to a difference in electronegativity of the bonded atoms. In $HCl$, the permanent dipole is shown by:
- A small positive charge on the $H$ atom, written as $\delta+$.
- A small negative charge on the $Cl$ atom, written as $\delta-$.
Polar Molecules
Non-symmetrical molecules such as $HCl$ are polar molecules due to the polar bonds. For symmetrical molecules however any dipoles may cancel out meaning it can be non-polar overall. $CCl_{4}$ is an example of this as each $C-Cl$ bond is polar however the molecule is symmetrical.
Electronegativity is measured using the Pauling Scale. The higher the electronegativity, the higher the value on the Pauling Scale. The electronegativity of an element can be predicted from the position on the periodic table:
- Reactive non-metallic elements such as $O$, $F$ and $Cl$ form compounds where they are the most electronegative atoms.
- Reactive metals such as $Na$ and $K$ form compounds where they are the least electronegative atoms.
Bonding Type
In a bond where the atoms have a slight difference in electroneagtivity, one of the atom attracts the atom slightly more than the other resulting in a polar covalent bond.
In a bond where the atoms have a large difference in electronegativity, one of the atoms would have captured the electrons forming an ionic bond.
Intermolecular Forces
Unlike ionic and covalent bonds, intermolecular bonds act between molecules. Intermolecular bonds are formed by small dipoles. There are three types of intermolecular forces:
Permanent dipole-dipole interactions
A permanent dipole-dipole is a weak attractive force between permanent dipoles in neighbouring polar molecules.
$$ H^{\delta +}-Cl^{\delta - } --------- H^{\delta +}-Cl^{\delta -} $$
Van der Waals Forces
Van der Waals forces are induced dipoles between neighbouring molecules. They exist between all simple covalent molecules.
These forces are caused by the movement of electrons. This movement generates an instantaneous dipole. This induced dipoles induces more dipoles in neighbouring molecules, which results in the formation of van der Waals' forces.
Van der Waals forces increase with the number of electrons. The stronger the van der Waals forces, the higher the boiling point of the molecules.
Hydrogen Bonding
Molecules containing either $O-H$ or $N-H$ bonds are polar with strong permanent dipoles. These permanent dipole-dipole interactions are called hydrogen bonds. For example, in water each oxygen has four bonds: two covalent bonds and two hydrogen bonds. The slightly negative $O^{\delta -}$ form bonds with the slightly positive $H^{\delta +}$.
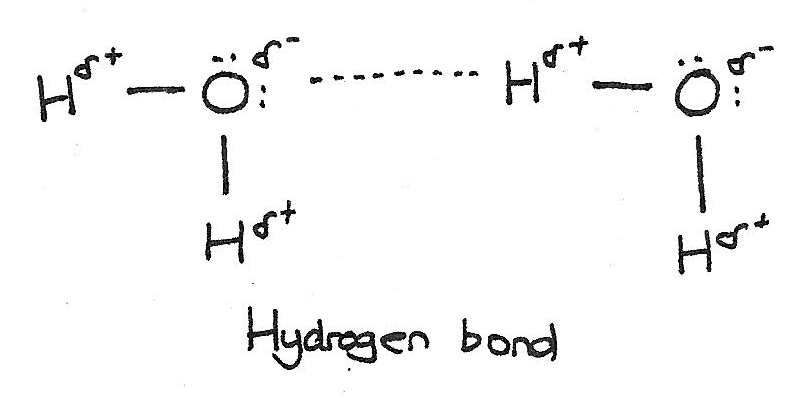
These hydrogen bonds give two unexpected properties of water:
- Ice is less dense than water. Usually, the solid is more dense however ice has an open lattice held together by hydrogen bonds. Upon melting, this structure collapses.
- Water has a relatively high boiling and melting point. As hydrogen bonds are stronger than van der Waals forces, more energy is required to break the bonding.
Metallic Bonding
The atoms in a solid metal are held together by metallic bonding. In metallic bonding, positive ions form an electrostatic attraction with electrons in a sea of delocalised electrons. Some of the properties are shown below:
- High melting and boiling points
This is due to the strong electrostatic attraction between the positive ions and sea of delocalised electrons. - Good electrical conductivity
This is because the delocalised electrons are able to move freely within the lattice. - High Malleability and Ductility
Ductility is the ability to be stretched and drawn out. Malleability is its ability to be hammered into shape.
Giant Ionic Lattice
Ionic bonds are formed by the transfer of electrons between a non metal and a metal. In a giant ionic lattice each ion is surrounded by oppositely charged ions. These ions attract each other forming a giant ionic lattice. Some of the properties are shown below:
- High melting and boiling points
Ionic compounds are solids at room temperature. A large amount of energy is needed to break the strong electrostatic attraction between the bonds, which gives the ionic compounds a high melting point. - Good Electrical Conductivity when molten
When solid, it forms a fixed lattice where no ions can move. When an ionic compound is melted or dissolved, the ions are free to move, and is able to conduct electricity. - Solubility
The ionic compound breaks down due to the polar water molecules which surround each atom.
The strength of the ionic lattice depends on the charge between the atoms. For example the electrostatic attraction between $Mg^{2+}$ and $O^{2-}$ is greater than between $Na^{+}$ and $Cl^{-}$.
Covalent Structures
Elements with covalent structures can have either of two structures:
Simple molecular lattice
These structures are made up from small, simple molecules such as $O_{2}$ and $N_{2}$. Molecules are held together through weak van der Waals' forces. The properties of simple molecular lattices are shown below:
- Low melting and boiling points
This is because the intermolecular bonds are weak van der Waals' forces so only a small amount of energy is needed to break the bonds. - Electrical conductivity
Simple covalent structures are non conductors of electricity because there are no charged particles free to move and conduct. - Solubility
Simple molecular structures are soluble in polar solvents as van der Waals' forces are able to form between the polar solvent and the molecular structure.
Giant Covalent Structures
This is where atoms are held together by strong covalent bonds in a lattice. Some of the properties are shown below:
- High melting points and boiling points
This is because high temperatures are needed to break the strong covalent bonds within the lattice. - Electrical conductivity
Giant covalent structures are non conductors of electricity as there are no free charged particles (except in graphite). - Solubility
Giant covalent structures are insoluble in both polar and non-polar solvents as the covalent bonds in the lattice are too strong to be broken.
Properties of Diamond
Diamond has a tetrahedral structure held together by strong covalent bonds throughout the structure which makes diamond hard. It is poor conductor of electricity however, as there are no delocalised electrons.
Properties of graphite
Graphite has a strong hexagonal layer structure but with weak van der Waals' forces between the layers. This makes it a good conductor of electricity as delocalised electrons are between the layers. Graphite is also soft as the layers are able to slide above each other.