Introduction to Crude Oil
What is crude oil?
Crude oil is a fossil fuel formed from decaying plants and animals that have decomposed over millions of years. Typically, crude oil is a mixture of over 150 different hydrocarbons, most of which are unbranched straight chain alkanes.
Fractional Distillation
Separation of the different hydrocarbon chains is done through fractional distillation:
- This takes place in a fractionating column.
- Crude oil is vaporised and passed through a column.
- The column is hotter towards the bottom than the top.
- When the gases reach a temperature lower than their boiling point, they condense and the fractions are removed.
- Short-chained hydrocarbons with low boiling points are extracted near the top of the column. Longer-chained hydrocarbons with higher boiling points condense nearer to the bottom.
Effect of chain length on boiling points
As the chain length increases, the boiling point increases. This is because in longer chained alkanes, there are more points of contact leading to more van der Waals' forces. More energy is needed to break the bonds, requiring more energy. This requires a higher temperature.
Effect of branching
A branched isomer has a lower boiling point than an unbranched isomer. This is because branched alkanes have fewer points of contact between the molecules, leading to fewer van der Waals' forces, requiring less energy to break the bonds.
Combustion of alkanes
Complete combustion:
$CH_{4(g)} + 2O_{2(g)}
\rightarrow CO_{2(g)} + 2H_{2}O_{(l)}$
Incomplete combustion:
$2CH_{4(g)} + 3O_{2(g)}
\rightarrow 2CO{(g)} + 4H_{2}O_{(l)}$
Cracking
Cracking is used to break down long-chained saturated hydrocarbons to form a mixture of shorter chained alkanes and alkenes. A catalyst of zeolite is used.
$C_{12}H_{26} \rightarrow C_{9}H_{20} + C_{3}H_{6}$
Isomerism
This is the process of converting straight chained alkanes into branched alkanes.
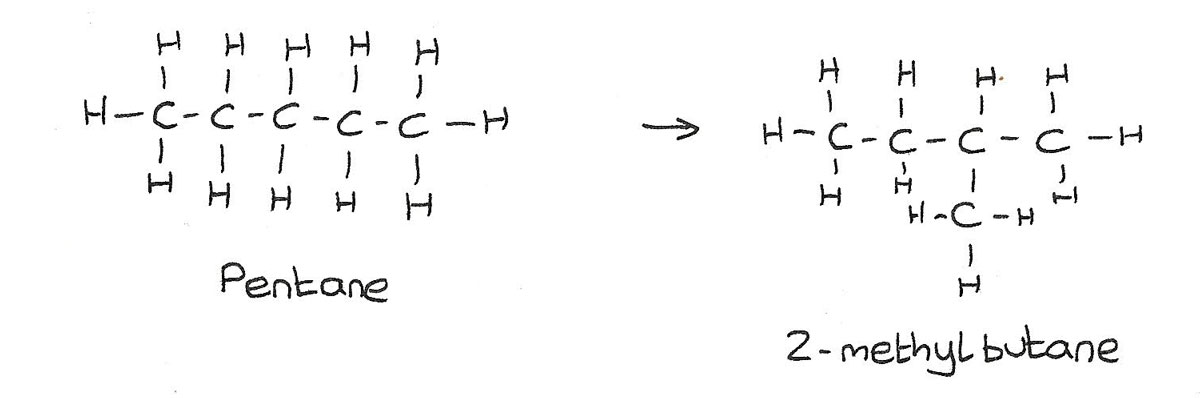
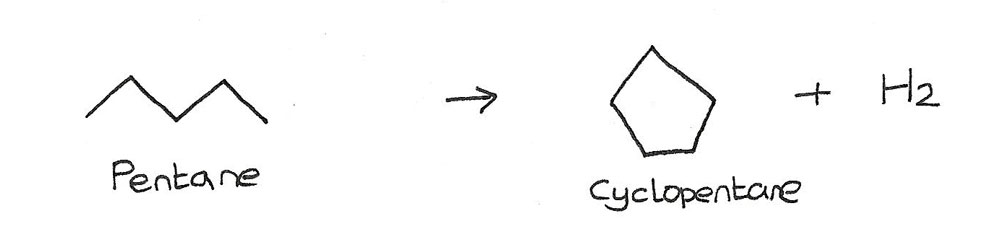
Reforming
This is the process of converting aliphatic (straight chain) alkanes into cyclic or aromatic alkanes.
Improving Fuels
How well a fuel burns is rated by its octane number. Fuels with higher octane ratings closer to 100 burn more efficiently. Branched and cyclic compounds promote more efficient combustion so they are used extensively used in fuels for car engines.
Use of crude oils
Good fuels need to be readily available, easily transportable and inexpensive to produce. In recent years, scientists have started to look for alternatives to using crude oil, due to growing concerns for the environment.
Burning hydrocarbons lead to an increase in atmospheric pollutants such as:
- Carbon monoxide - a toxic gas formed by incomplete combustion in the internal combustion engine.
- Carbon dioxide - a major contributor to global warming via the greenhouse effect.
- Nitrogen Oxides - contributor to acid rain and destruction of forests.
Global Warming
Burning fuels release carbon dioxide and other greenhouse gases into the atmosphere. These gases prevent heat from escaping which lead to increased temperatures on Earth. These warmer conditions can lead climate change with heavier rain and more violent storms. In addition, ice caps are threatened, with a consequential rise in sea levels and more localised flooding.
Biofuels
A biofuel is a fuel that is derived from living material such as plants, or animal waste.