Aldehydes and Ketones
Ketones and aldehydes both contain the carbonyl functional group, $C=O$.
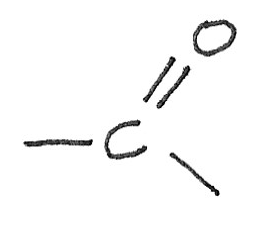
The overlap of p-orbital electrons above and below the plane of the carbon and the oxygen atoms result in the formation of a $\pi$ bond. The sideways overlap between the $C$ and $O$ orbitals form a $\sigma$ bond.
Difference with Alkenes
While alkenes have a double bond between two carbon atoms, the double bond in carbonyls is between a carbon atom and an oxygen atom. In this arrangement, the oxygen atom is more electronegative than a carbon atom. This creates a dipole in the $C=O$ bond as electrons are attracted more to the oxygen atom.
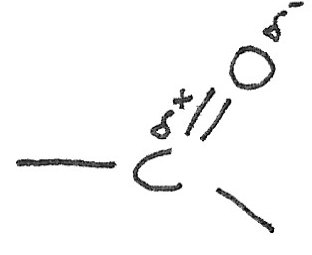
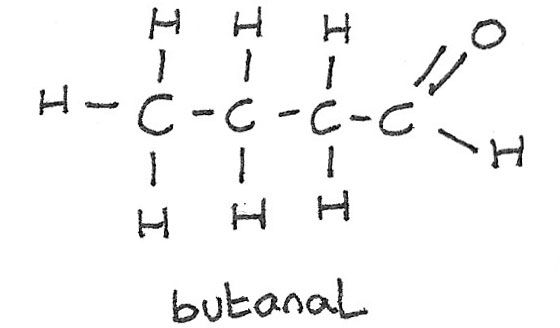
Naming Aldehydes
In aldehydes the carbonyl functional group is always at the end of the carbon chain. This gives the molecule the -al suffix.
Naming Ketones
The oxygen atom in ketones can be attached to any of the carbon atoms not at the end of the hydrocarbon chain. Ketones have the -one suffix. It is important to use the smallest possible carbon atom number when naming the molecule.
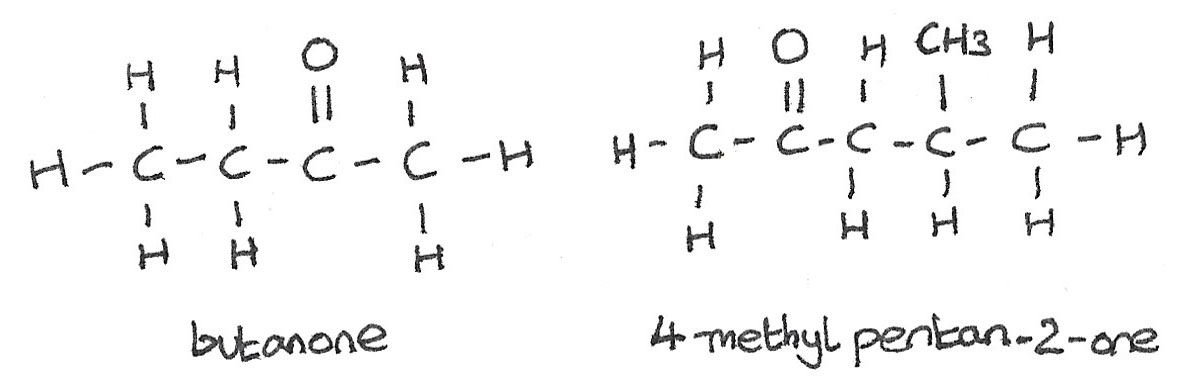
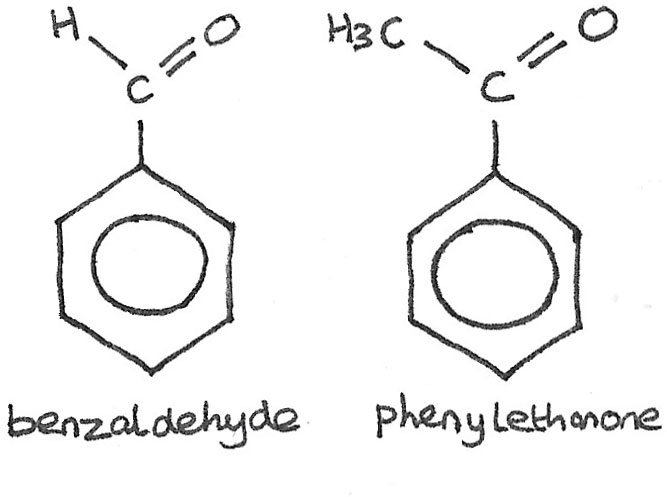
Aromatic Ketones and Aldehydes
Aromatic ketones and aldehydes are compounds that contain both a carbonyl group and a benzene ring. The simplest aromatic aldehyde is benzaldehyde and the simplest aromatic ketone is phenylethanone.
Oxidation of Alcohols
Primary and secondary alcohols can be oxidised using an oxidising agent such as acidified potassium dichromate ($K_{2}Cr_{2}O_{7}$), which supplies the ions $H^{+}/Cr_{2}O_{7}^{~2-}$. Sulphuric acid ($H_{2}SO_{4}$) is used as a catalyst. During the reaction, the solution turns from orange to green.
Primary Alcohols
These are oxidised first to aldehydes, then oxidised further to carboxylic acids.
$$ CH_{3}CH_{2}OH + [O] \rightarrow CH_{3}CHO + H_{2}O \\
CH_{3}CHO + [O] \rightarrow CH_{3}COOH $$
In order to extract an aldehyde, the product must be distilled off as it is produced. To form the carboxylic acid, the reaction mixture must be heated under reflux.
Secondary Alcohols
Due to the positioning of the $C=O$ bond, secondary alcohols can only be oxidised to form ketones.
$$ CH_{3}CHOHCH_{3} + [O] \rightarrow CH_{3}COCH_{3} + H_{2}O $$
Tertiary Alcohols
Tertiary alcohols are resistant to oxidation. No reaction occurs and therefore the oxidising agent remains orange.
Reduction of Ketones and Aldehydes
Carbonyl compounds can be reduced to alcohols by warming with a suitable reducing agent such as sodium borohydride ($NaBH_{4}$). Water is often used as the solvent.
Aldehydes
Aldehydes are reduced to primary alcohols. In this example, butanal is reduced to butan-1-ol.
$$CH_{3}CH_{2}CH_{2}CHO + 2[H] \rightarrow CH_{3}CH_{2}CH_{2}CH_{2}OH $$
Ketones
Ketones are reduced to secondary alcohols. In this example, butanone is reduced to butan-2-ol.
$$CH_{3}CH_{2}COCH_{3} + 2[H] \rightarrow CH_{3}CH_{2}CH(OH)CH_{3} $$
Nucleophilic Addition Mechanism
Ketones and aldehydes react with $NaBH_{4}$ in a nucleophilic addition reaction. The $BH_{4}^{-}$ ions acts as a source of the hydride ions, $H^{-}$. These hydride ions act as the nucleophile.
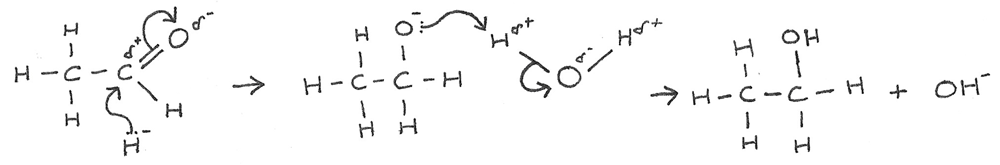
- There is a nucleophilic attack by the hydride ion on the slightly positive carbon atom.
- The lone pair of electrons from the ${:H^{-}}$ ion forms a dative covalent bond with the carbon atom.
- At the same time the $\pi$ bond in the $C=O$ bond breaks to produce a negatively charged intermediate.
- The intermediate then donates an electron pair to a hydrogen atom in $H_{2}O$.
- This results in the formation of an alcohol and hydroxide ion ($OH^{-}$).
Chemical Testing of Carbonyl Compounds
Brady's Reagent
Ketones and aldehydes can be detected by using a solution known as Brady's Reagent. Brady's Reagent is a solution of 2,4-dinitrophenylhydrazine ($2,4-DNP$) in a mixture of methanol and sulphuric acid.
Upon adding Brady’s Reagent to an aldehyde or ketone, an orange precipitate is formed, confirming the presence of a $C=O$ bond. This precipitate is known as a 2,4-dinitrophenylhydrazone derivative. There is no visible difference between the reactions between aldehydes and ketones.
Further Tests with DNP Derivative
After using Brady’s reagent, a dinitrophenylhydrazone derivative is formed. This solid can be filtered and recrystallised to produce a purified sample. The melting point of this purified product is measured and recorded. The results can be compared to a known database in order to determine the original aldehyde or ketone.
Tollens' Reagent
Once a compound has been identified as a carbonyl, it can be further identified using Tollens' Reagent. This is a weak oxidising agent that can distinguish between ketones and aldehydes.
Aldehydes are easily oxidised to carboxylic acids, forming a "silver mirror" effect. Ketones and carboxylic acids cannot be oxidised further so no reaction occurs.
Tollens' Reagent can be made by heating aqueous sodium hydroxide with aqueous silver nitrate until a brown precipitate is formed. Dilute aqueous ammonia is added until the precipitate dissolves. This produces Tollens' Reagent, a colourless oxidising agent. The oxidising species is the silver ion, $Ag^{+}_{(aq)}$.
| Aldehyde | Ketone | Carboxylic Acid | |
|---|---|---|---|
| Brady's Reagent | Orange Precipitate | Orange Precipitate | No Reaction |
| Tollens’ Reagent | Silver Mirror | No Reaction | No Reaction |