Halogenoalkanes
Halogenoalkanes are compounds in which a halogen atom has replaced at least one of the hydrogen atoms in an alkane chain. They have the general formula: $C_{n}H_{2n+1}X$, where $X$ is a halogen atom.
Naming Halogenoalkanes
Their name is based on the length of the longest alkane chain with any halogen groups and positions indicated. If there are more than one halogen atom then they are listed alphabetically.
- fluoro- ($F$)
- chloro ($Cl$)
- bromo- ($Br$)
- iodo- ($I$)
Reactivity of the halogenoalkanes
Halogenoalkanes contain a polar carbon-halogen bond. This polarity arises from the different electronegativity of the carbon and halogen atoms.
- Halogens are more electronegative than carbon atoms.
- The bonded electron pair is attracted more towards the halogen atom than towards the carbon atom which results in a polar bond.
The electronegativity of the halogen decreases down the group, resulting in a decrease in polarity of the carbon-hydrogen bond from fluorine to iodine.
The electron deficient carbon atom in halogenoalkanes attracts nucleophiles. This allows halogenoalkanes to react with nucleophiles in substitution reactions.
Hydrolysis of Halogenoalkanes
When halogenoalkanes react with an aqueous solution of hot hydroxide ions, a nucleophilic substitution reaction occurs which gives an alcohol. This reaction is called hydrolysis.
$$CH_{2}ClCH_{3} + NaOH \rightarrow CH_{2}OHCH_{3} + Cl^{-}$$
Reactions of Halogenoalkanes
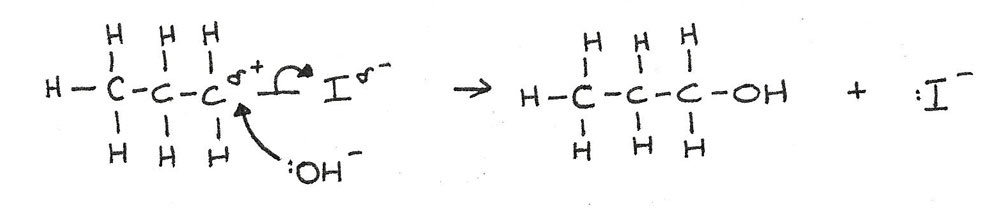
In nucleophilic substitution, an atom or group of atoms is replaced by a nucleophile (an electron pair donor). During hydrolysis, the halogen atom is replaced by the hydroxide ion:
The hydroxide ion has a lone pair of electrons. These are attracted and donated to the electron-deficient carbon atom in the halogenoalkane. This is known as nucleophilic attack.
The donation of the electron pair leads to the formation of a new covalent bond between the oxygen atom of the hydroxide ion and the carbon atom.
The carbon-halogen bond breaks by heterolytic fission. Both of the bonded electrons move to the halogen, forming a halide ion.
This mechanism for nucleophilic substitution of 1-iodopropane is shown below:
Rate of Hydrolysis
The rates of hydrolysis for different halogenalkanes can be determined using the following experiment:
- The halogenoalkane is heated with aqueous silver nitrate and ethanol.
- The water in the mixture acts of the nucleophile.
- The ethanol acts as a common solvent, ensuring that the halogenoalkane and aqueous silver nitrate mix together and react.
- During the reaction, halides are formed. Shown below is the hydrolysis of chloroethane:
$$CH_{3}CH_{2}Cl_{(aq)} + H_{2}O_{(l)} \rightarrow CH_{3}CH_{2}OH_{(aq)} + H^{+}_{(aq)} + Cl^{-}_{(aq)}$$
The aqueous silver nitrate, $AgNO_{3(aq)}$ reacts with any halide ions present, forming a precipitate.
- Chloroalkane forms $AgCl_{(s)}$ a white precipitate.
- Bromoalkane forms $AgBr_{(s)}$ a cream precipitate.
- Iodoalkane forms $AgI_{(s)}$ a yellow precipitate.
The rate of hydrolysis can be calculated by doing: $$ rate\,of \,hydrolysis = \frac {1}{time} $$
Bond Enthalpy in Hydrolysis
The carbon-iodine bond is the weakest of the halogenoalkanes, therefore this more readily reacts and is broken the most easily. The bond enthalpy decreases down the group.
Halogenoalkanes in the environment
Many of halogen containing polymers can be produced such as PTFE from the polymerisation of tetrafluorethene and poly(vinyl chloride).
- The properties of the polymers are influenced by the strength of the carbon-halogen bond.
- As carbon-flourine bonds are very strong, this makes PTFE inert and resistant to chemical attack. In addition, PTFE has good heat resistance, electrical insulating properties and non-stick properties which makes it a popular choice for coating pans among other uses.
- PVC is used in many appliances such as drainpipes, plastic window frames and packaging.
Chlorofluorocarbons
Chlorofluorocarbons are halogenoalkanes containing only carbon, fluorine and chlorine atoms. Developed by Thomas Midgley in 1929, they were popular as refrigerants and propellants due to their non-toxic, unreactive and non-flammable properties.
Trouble with CFCs
The stability of CFCs arises from the strength of the carbon-halogen bonds, however this produces a problem.
- Up to the stratosphere, they remain stable, where they break down in the presence of UV radiation to form chlorine radicals.
- These radicals catalyse the breakdown of the ozone layer.
- Ozone absorbs much of the UV from the sun, preventing the radiation from reaching the surface.
- This depletion allows harmful UV radiation to reach the surface, increasing the risk of skin cancers.
As an alternative, hydrofluorocarbons have now been developed. Despite the non-flammable and non-toxic properties,they still deplete the ozone layer so are widely considered as a short term replacement.