Chromatography
Chromatography is an analytical technique used to separate components with similar physical properties from a mixture. In chromatography, a mobile phase sweeps a mixture over a fixed stationary phase. The greater the interaction between the stationary phase and the components in the mobile phase, the slower the components move. The difference in speed between components results in the separation.
The two main types of chromatography are:
- Thin-layer chromatography where the stationary phase is a solid and the mobile phase is a liquid.
- Gas chromatography where the stationary phase is either a solid or liquid and the mobile phase is a gas.
Separation Methods
- A solid stationary phase separates the components through adsorption, where gas or liquid molecules are held on the surface of a solid. The stronger the adsorption of a component onto the stationary phase, the slower it moves.
- A liquid stationary phase causes separation due to the relative solubility of the components. The components which dissolve more readily are slowed down more than components with lower solubility.

Thin Layer Chromatography
Thin layer chromatography is used for separating a mixture into its components with the use of a solid stationary phase and a liquid mobile phase.
- The solid stationary phase is a thin layer of adsorbent such as silica gel ($SiO_{2}$) or alumina ($Al_{2}O_{3}$) coated onto a flat inert support of plastic or glass. This is called the TLC plate.
- The liquid mobile phase is a liquid solvent which moves vertically up the TLC plate.
Producing the Chromatogram
A chromatogram is a visible record showing the result of the separation. Thin layer chromatography is achieved by the adsorption of the components onto the solid stationary phase. The components which bind to the adsorbent more strongly move slower up the plate.
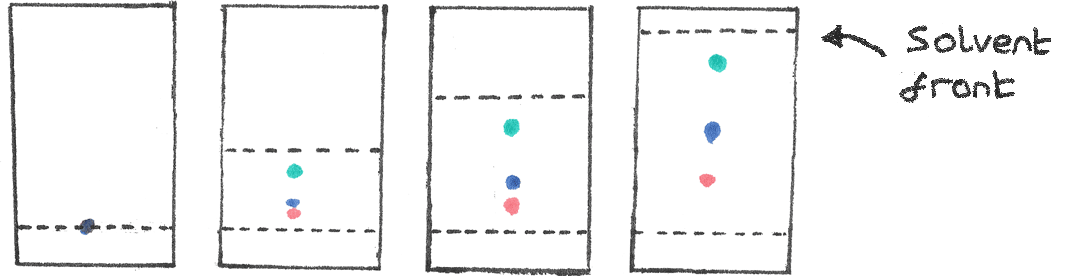
The diagram above shows the movement of the separated components as the solvent rises up the plate. The method for thin layer chromatography is shown below:
- First a sample of the mixture is dissolved into aqueous solution. A small drop is then placed close to the bottom of the TLC plate and allowed to dry.
- A horizontal pencil line should be drawn to indicate the starting position of the sample. Ink should not be used as the ink would separate on the TLC plate.
- The TLC plate should be placed into the beaker with a shallow layer of solvent below the sample line. The beaker should then be sealed in order to saturate the space in the jar with solvent vapour. This slows down the evaporation of the solvent, reducing solvent loss.
- The solvent will then rise up the plate due to capillary action. Along with the solvent, the components in the sample are also swept vertically up the TLC plate. Once the solvent has nearly reached the top of the plate, the plate should be removed and allowed to dry.
- A pencil line should be drawn at the maximum height reached by the solvent. The generated chromatogram should show spots of the separated mixture.
Analysing the Chromatogram
Each separated component appears as a spot on the TLC plate. Sometimes the spots may be colourless, in which case a method should be used to locate the spots:
- A locating agent can be reacted with the components, forming a visible coloured product.
- The stationary phase often contains a substance which fluoresces under ultraviolet light. UV light can be shined onto the plate which will reveal darker spots where the components are located.
Once the positions of the separated components have been determined, the TLC chromatograms can be interpreted through the use of $R_{f}$ values. These indicate how far the component has travelled up the TLC plate.
$$ R_{f} = \frac{\text{distance moved by component}}{\text{distance moved by solvent front}} $$
The value of $R_{f}$ has no units and will always be between $0$ and $1$. In the diagram below the distance moved by the solvent front is represented by $y$ while the distance moved by the component represented by $x$.
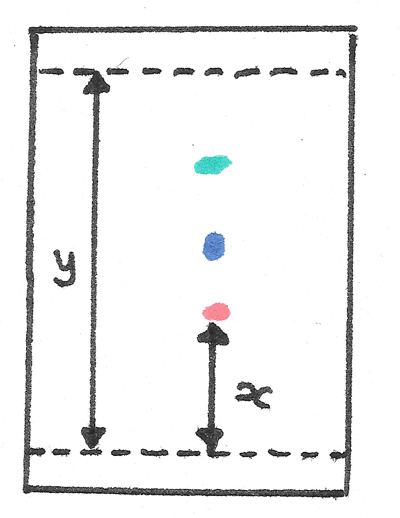
If the chromatography was repeated, the same $R_{f}$ value would be determined. Unknown substances can be identified by comparing them to known values.
Limitations of TLC
- Similar compounds often have similar $R_{f}$ values so it can be difficult to accurately determine a compound.
- Unknown compounds have no $R_{f}$ values for reference.
- Sometimes it can be difficult to find a solvent that separates all the components in a mixture. If the components are too soluble in the solvent, they are just washed up the TLC plate by the solvent. If the compounds only have little solubility they will hardly move.
Gas Chromatography
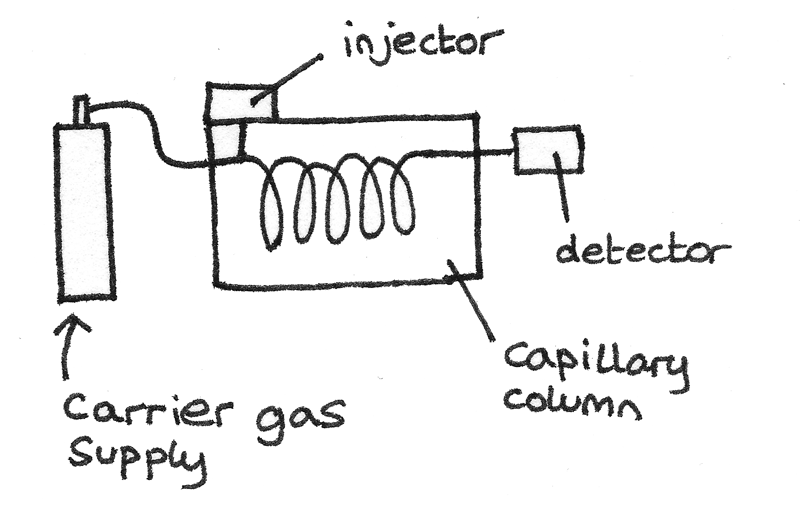
Gas chromatography is a technique used to separate volatile compounds. It takes place in an instrument called a gas chromatograph.
- A capillary tube acting as an inert solid support, holds the stationary phase. This tube is wound into a coil and inserted into a thermostatically controlled oven. Together it is called a chromatography column.
- The stationary phase can either be a liquid or a solid. As a liquid, long chain alkanes with high boiling points are often used. Suitable solid stationary phases include silicon polymers.
- The mobile phase is an inert carrier gas such as nitrogen or helium. The sample gas is vaporised into this phase and passed through the capillary column.
Producing the Chromatogram
- The mixture is vaporised into the gas chromatograph. The mobile phase carries the mixture through the capillary column.
- As the components interact with the stationary phase they are slowed down by different amounts, resulting in the separation. If the stationary phase is solid, the components are slowed by adsorption. If the stationary phase is liquid, the greater the solubility of the gas into the liquid, the more they are slowed down.
- Each compound leaves the column and is detected at different times. A computer processes these results to produce a gas chromatogram.
Retention Time
The retention time is the time taken for a component to pass from the column inlet to the detector. As each compound is slowed by a different amount, each compound has a unique retention time. The graph below shows a chromatogram of alcohols and carbonyl compounds in a sample of blood.
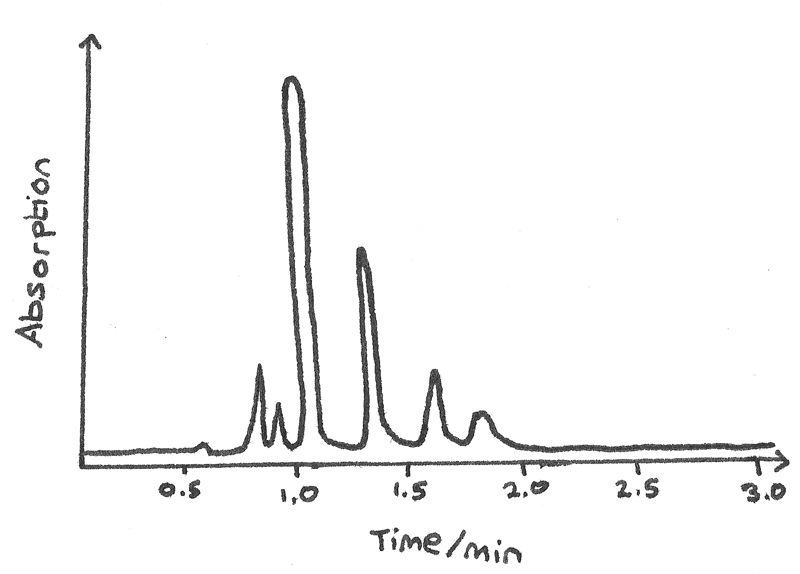
- The individual peaks correspond to different compounds. For example the tallest peak at $1.0~min$ matches with ethanol while the small peak closest to $2.0~min$ corresponds to propan-1-ol. This allows for the identification of unknown compounds by matching results to a known database of retention times.
- The area under each peak is proportional to the amount of compound in the sample.
- The number of peaks is equal to the number of components in a compound with different retention times.
An everyday use of gas chromatography is in breathalysers to determine the amount of alcohol in the blood.
Limitations of Gas Chromatography
- Many chemicals could have very similar retention times, peak shapes and detector responses. Gas chromatography is therefore often used alongside mass spectroscopy to more positively identify a compound.
- Unknown compounds have no retention times for reference.
- Some substances may not be separated and detected as they are hidden by another substance of higher concentration with the same retention time.
GC-MS
Gas chromatography and mass spectrometry can be combined into a powerful analytical tool for the identification of compounds. This process is called gas chromatography-mass spectrometry or GC-MS.
- Gas molecules are first separated through gas chromatography. The retention times can provide an initial identification of the molecule.
- The separated components can be directed into a mass spectrometer where a mass spectrum is produced with molecular ion peaks and fragment ion peaks.
- The generated mass spectrum can be analysed and compared with a spectral database. As each compound has a unique mass spectrum, mass spectrometry allows for the positive identification of each component in a mixture.
Uses of GC-MS
- Forensics: GC-MS can be used to detect particular compounds or traces of illegal substances in a forensic sample.
- Environmental analysis: GC-MS can be used to analyse pollutants in the environment such as in waste water. It can also be used to detect pesticides in food.
- Airport Security: GC-MS techniques can be used in security systems to detect the presence of explosives in luggage and on people.
- Space Probes: GC-MS can be used to identify unknown compounds on the surface of or in the atmosphere of other planets.