Synthesis
Organic synthesis requires the knowledge of chemical reactions and conditions listed below.
Alkane → Halogenoalkane
Reaction mechanism: Free radical substitution
Conditions: UV light
Reactants: $X_{2}$
Example: $$ CH_{4} + Cl_{2} \rightarrow CH_{3}Cl + HCl $$
Halogenoalkane → Alcohol
Reaction mechanism: Nucleophilic substitution
Conditions: Heated under reflux
Reactants: $NaOH$
Example: $$ CH_{3}Cl + NaOH \rightarrow CH_{3}OH + NaCl $$
Halogenoalkane → Primary amine
Reaction mechanism: Nucleophilic substitution
Conditions: Ethanol as solvent, Warm
Reactants: Excess $NH_{3}$
Example: $$ CH_{3}CH_{2}Cl + NH_{3} \rightarrow CH_{3}CH_{2}NH_{2} + HCl $$
Primary amine → Secondary amine
Reaction mechanism: Nucleophilic substitution
Conditions: Warm
Reactants: Halogenoalkane
Example: $$ CH_{3}CH_{2}NH_{2} + CH_{3}CH_{2}Cl \rightarrow (CH_{3}CH_{2})_{2}NH + HCl $$
Secondary amine → Tertiary amine
Reaction mechanism: Nucleophilic substitution
Conditions: Warm
Reactants: Halogenoalkane
Example: $$ (CH_{3}CH_{2})_{2}NH + CH_{3}CH_{2}Cl \rightarrow (CH_{3}CH_{2})_{3}N + HCl $$
Alkene → Alkane
Reaction type: Hydrogenation
Conditions: Nickel ($Ni$) catalyst, $150°C$
Reactants: $H_{2}$ gas
Example: $$H_{2}C{=}CH_{2} + H_{2} \rightarrow CH_{3}CH_{3}$$
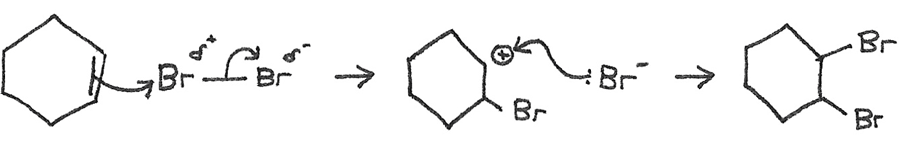
Alkene → Dibromoalkane
Reaction mechanism: Electrophilic addition
Conditions: Room temperature
Reactants: $Br_{2}$
Example: $$H_{2}C{=}CH_{2} + Br_{2} \rightarrow CH_{2}BrCH_{2}Br $$
Mechanism:
Alkene → Alcohol
Reaction type: Hydration
Conditions: Phosphoric acid catalyst, $300°C$ and $60~atm$
Reactants: Steam ($H_{2}O_{(g)}$)
Example: $$H_{2}C{=}CH_{2} + H_{2}O_{(g)} \rightarrow CH_{3}CH_{2}OH$$
Alcohol → Alkene
Reaction type: Dehydration
Conditions: Concentrated $H_{2}SO_{4}$ catalyst, heated under reflux
Example: $$ CH_{3}CH_{2}OH \rightarrow H_{2}C=CH_{2} + H_{2}O$$
Primary alcohol → Aldehyde
Reaction type: Oxidation
Conditions: Concentrated sulphuric acid ($H_{2}SO_{4}$), distillation
Reactants: $K_{2}Cr_{2}O_{7}$
Equation: $$ CH_{3}CH_{2}OH + [O] \rightarrow CH_{3}CHO + H_{2}O $$
Primary alcohol → Carboxylic acid
Reaction type: Oxidation
Conditions: Concentrated sulphuric acid ($H_{2}SO_{4}$), reflux
Reactants: $K_{2}Cr_{2}O_{7}$
Equation: $$ CH_{3}CH_{2}OH + 2[O] \rightarrow CH_{3}COOH + H_{2}O $$
Secondary alcohol → Ketone
Reaction type: Oxidation
Conditions: Concentrated sulphuric acid ($H_{2}SO_{4}$)
Reactants: $K_{2}Cr_{2}O_{7}$
Equation: $$ CH_{3}CHOHCH_{3} + [O] \rightarrow CH_{3}COCH_{3} + H_{2}O $$
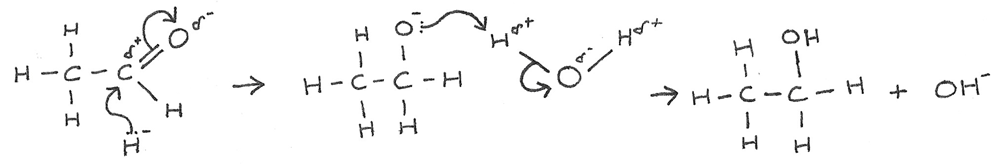
Aldehyde → Alcohol
Reaction mechanism: Nucleophilic addition
Conditions: Room temperature
Reactants: $NaBH_{4(aq)}$
Example:
$$ CH_{3}CH_{2}CH_{2}CHO + 2[H] \rightarrow CH_{3}CH_{2}CH_{2}CH_{2}OH $$
Mechanism:
Ketone → Alcohol
Conditions: Room temperature
Reactants: $NaBH_{4(aq)}$
Example:
$$ CH_{3}COCH_{3} + 2[H] \rightarrow CH_{3}CHOHCH_{3} $$
Carboxylic acid → Carboxylate salt
Conditions: Alkaline conditions
Reactants: $NaOH_{(aq)}$ / $Na_{(s)}$ / $Na_{2}CO_{3(s)}$
$$ CH_{3}COOH + NaOH \rightarrow CH_{3}COO^{-}Na^{+} + H_{2}O \\
CH_{3}COOH + Na \rightarrow CH_{3}COO^{-}Na^{+} +\frac{1}{2}H_{2} \\
2CH_{3}COOH_{(aq)} + Na_{2}CO_{3(s)} \rightarrow 2CH_{3}COO^-Na_{(aq)}^{+} + CO_{2(g)} + H_2O_{(l)} $$
Amine → Alkylammonium salt
Reactants: $HCl_{(aq)}$
Example:
$$ CH_{3}CH_{2}NH_{2(aq)} + HCl_{(aq)} \rightarrow CH_{3}CH_{2}NH_{3}^{+}Cl^{-}_{(aq)} $$
Carboxylic acid + Alcohol → Ester
Reaction type: Esterification
Conditions: Concentrated sulphuric acid ($H_{2}SO_{4}$) catalyst, heated under reflux
Reactants: Alcohol with carboxylic acid
Example:
$$ CH_{3}CH_{2}COOH + CH_{3}CH_{2}OH \rightarrow CH_{3}CH_{2}COOCH_{2}CH_{3} + H_{2}O $$
Acid anhydride + Alcohol → Ester
Reaction type: Esterification
Reactants: Acid anhydride with alcohol
Example:
$$ (CH_{3}CO)_{2}O + CH_{3}OH \rightarrow CH_{3}COOCH_{3} + CH_{3}COOH $$
Ester → Carboxylic acid + Alcohol
Reaction type: Acid hydrolysis
Conditions: Dilute hydrochloric acid ($HCl$), heated under reflux
Reactants: Ester and water
Example:
$$ CH_{3}COOCH_{2}CH_{2}CH_{3} + H_{2}O \rightleftharpoons CH_{3}COOH + CH_{3}CH_{2}CH_{2}OH $$
Ester → Carboxylate salt + alcohol
Reaction type: Alkaline hydrolysis
Conditions: Alkaline conditions
Reactants: $KOH$
Example:
$$ CH_{3}CH_{2}COOCH_{2}CH_{3} + KOH \rightarrow CH_{3}CH_{2}COO^{-}K^{+} + CH_{3}CH_{2}OH $$
Benzene → Nitrobenzene
Reaction type: Nitration
Conditions: Concentrated sulphuric acid ($H_{2}SO_{4}$) catalyst and $50°C$
Reactants: Concentrated nitric acid, $HNO_{3}$
Equation:
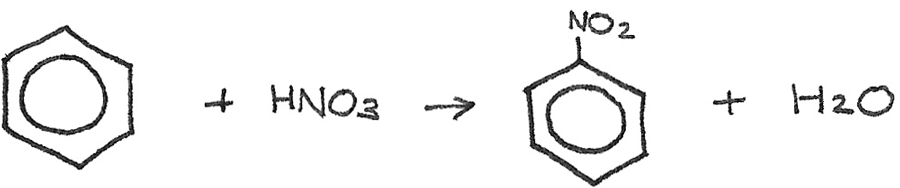
Mechanism:
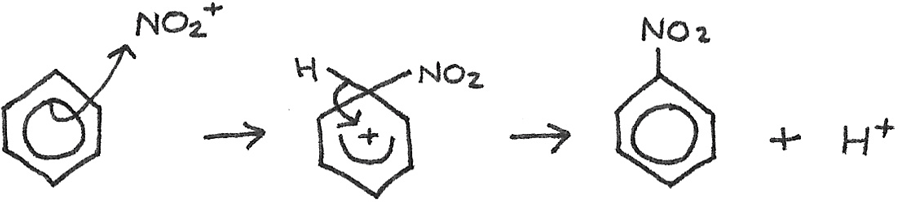
The sulphuric acid is used to generate an electrophile, which is the nitronium ion ($NO_2^{+}$) from the nitric acid:
$$H_{2}SO_{4} + HNO_{3} \rightarrow HSO_{4}^{-} + NO_{2}^{+} + H_{2}O$$
Benzene → Halobenzene
Reaction mechanism: Electrophilic substitution
Conditions: Halogen carrier ($FeBr_{3}$ or $AlBr_{3}$)
Equation:
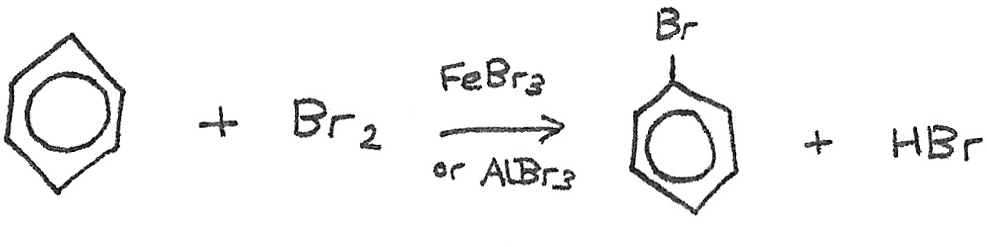
Mechanism:
A $Br^{+}$ ion is formed as below:
$$ Br_{2} + FeBr_{3} \rightarrow Br^{+} + FeBr_{4}^{-}$$The electrophile now reacts with the benzene in the following mechanism.
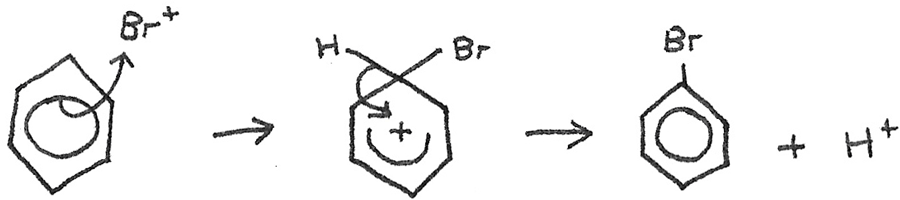
The $H^{+}$ now reacts with the $FeBr_{4}^{-}$ to reform $HBr$ and $FeBr_{3}$.
$$ H^{+} + FeBr_{4}^{-} \rightarrow FeBr_{3} + HBr $$
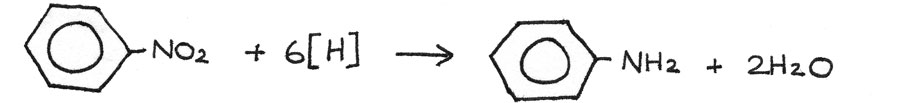
Nitrobenzene → Phenylamine
Reaction type: Reduction
Conditions: Tin ($Sn$) catalyst, heated under reflux.
Reactants: Concentrated hydrochloric acid ($HCl$)
Equation:
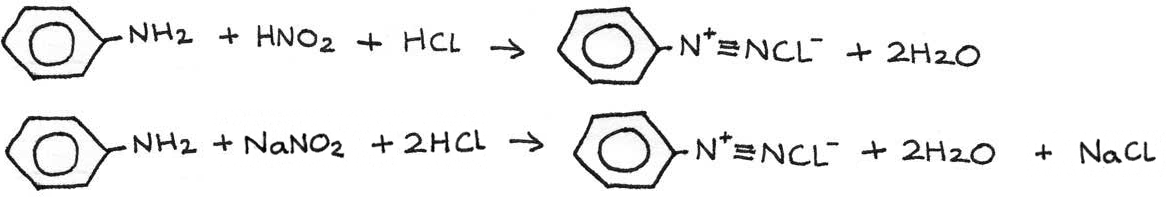
Phenylamine → Diazonium Salt
Reaction type: Diazotisation
Conditions: Less than $10°C$
Reactants: $HNO_{2}$ and $HCl$ or $NaNO_{2}$ and $HCl$
Equation:
Diazonium Salt → Azo Compound
Reaction type: Coupling
Conditions: Alkaline conditions
Reactants: Sodium hydroxide ($NaOH$) and phenol
Equation:

Phenol → Sodium Phenoxide
Reactants: $NaOH_{(aq)}$ / $Na$
Equation:
$$C_{6}H_{5}OH + NaOH \rightarrow C_{6}H_{5}O^{-}Na^{+} + H_{2}O \\
2C_{6}H_{5}OH + 2Na \rightarrow 2C_{6}H_{5}O^{-}Na^{+} + H_{2} $$
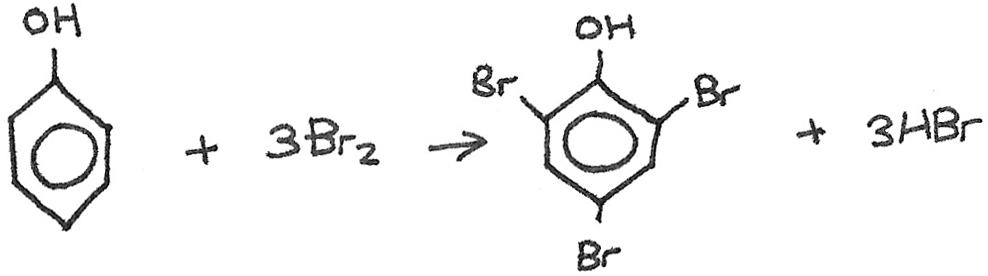
Phenol → 2,4,6-Tribromophenol
Reaction type: Halogenation
Reactants: $Br_{2}$ and phenol
Equation: