Polymers
Condensation Polymerisation
Condensation polymerisation is the joining of two monomer units with the elimination of a small molecule such as water or $HCl$. Condensation polymerisation requires monomers with two different functional groups. A functional group on one of the monomer units bonds with a different functional group on the other unit.
Polyesters
A polyester is a polymer where the monomers are held together through ester linkages. In a polyester, monomers must have a carboxyl group ($-COOH$) and a hydroxyl group ($-OH$). There are two types of polyester.
Different Types of Monomer Units
With this method, one monomer is a dicarboxylic acid with two $-COOH$ groups while the other is a diol with two $-OH$ groups. The general formation is shown below:
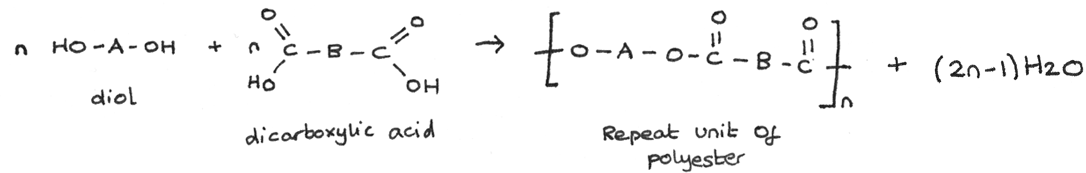
An example of this condensation reaction is the formation of Terylene which is made from the monomers ethane-1,2-diol and benzene-1,4-dicarboxylic acid.
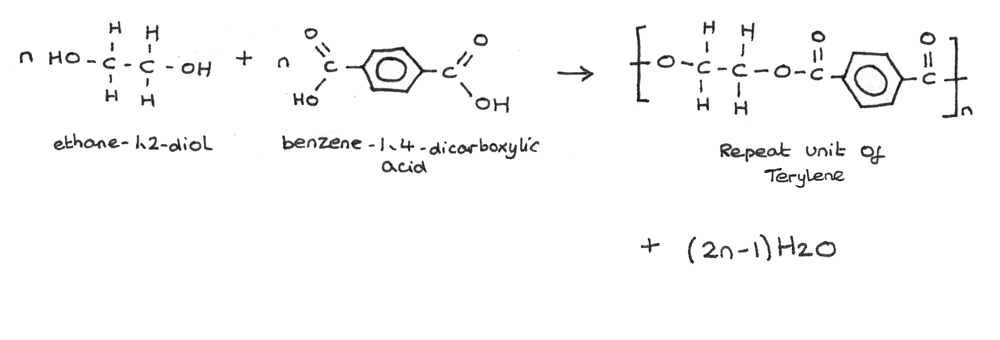
One type of Monomer Unit
Polyesters can also be formed from hydroxycarboxylic acids, which contain both the carboxyl group, $-COOH$ and a hydroxyl group, $-OH$. The general formation is shown below:
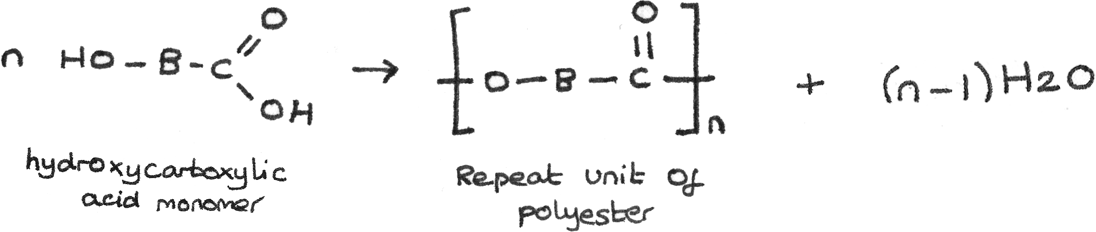
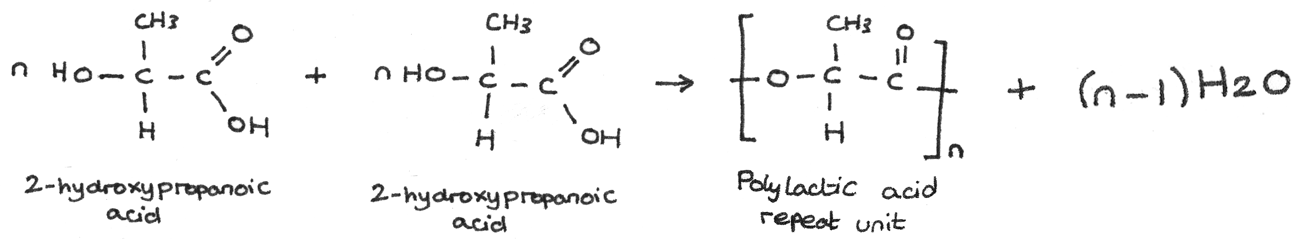
An example of this type of condensation reaction is the formation of polylactic acid (PLA) from the monomer unit 2-hydroxypropanoic acid. During this polymerisation, an ester bond forms between the carboxyl group on one unit and a hydroxyl group on another unit. In forming this ester linkage, water is eliminated.
Polyamides
Like polyesters, polyamides can be formed from two types of monomer units or just one type of monomer unit. In forming a polyamide, the monomer units are joined through amide linkages, with the elimination of water. In a polyamide, the monomers must have a carboxyl group ($-COOH$) and an amine group ($-NH_{2}$).
Different Types of Monomer Units
Polyamides can be made by reacting together one monomer which is a dicarboxylic acid, with another monomer which is a diamine. The general formation is shown below:
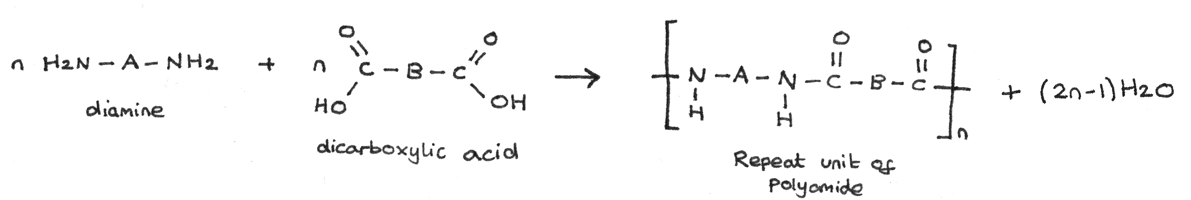
An example of this type of condensation polymerisation is the formation of Nylon-6,6. This is made from the monomers 1,6-diaminohexane and hexanedioic acid.
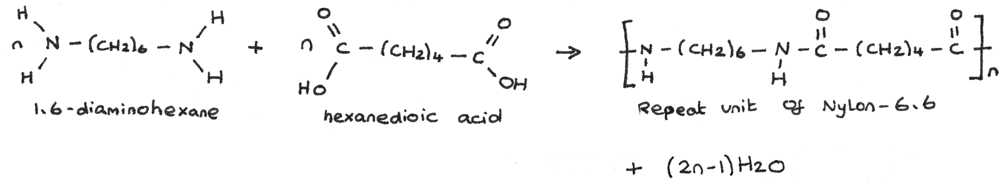
Nylon-6,6 is often used in clothing. Kevlar is another material used in clothing which is synthesised through condensation polymerisation. Kevlar is a material used for protective clothing such as bulletproof vests and helmets, due to its high strength and fire resistance.
Kevlar is formed from the polymerisation of benzene-1,4-dioic acid and benzene-1,4-diamine.
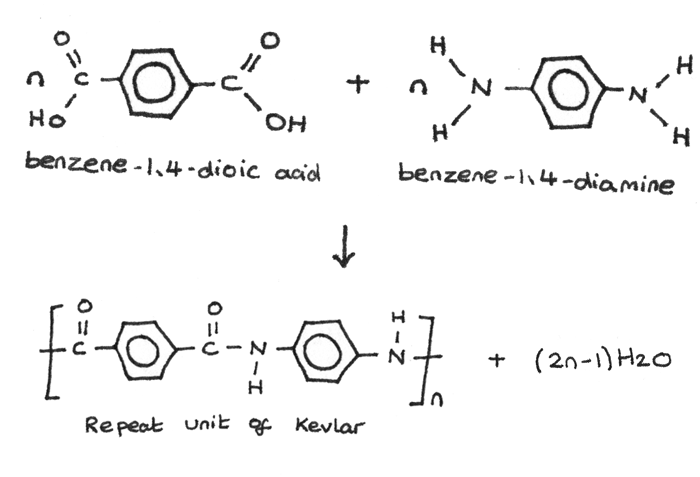
One type of Monomer Unit
Polyamides can also be formed from amino acids, which contain both the amine group ($-NH_{2}$) and a carboxyl group ($-COOH$). Polypeptides and proteins are polyamides formed from one type of monomer unit. The general formation is shown below:
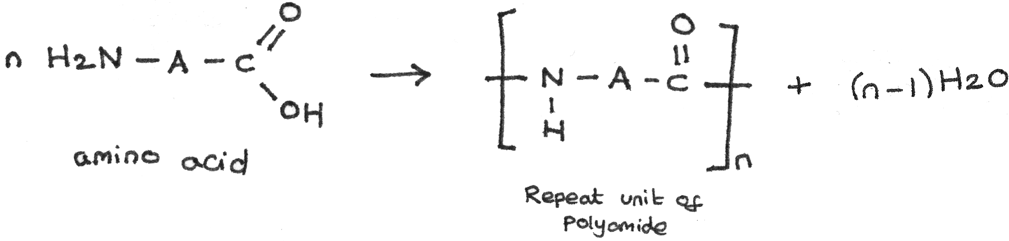
Addition Polymerisation
Addition polymerisation is the process in which alkene monomers undergo addition polymerisation to produce a saturated polymer chain. Addition polymers are made from only one type of monomer. The polymer is the only product.
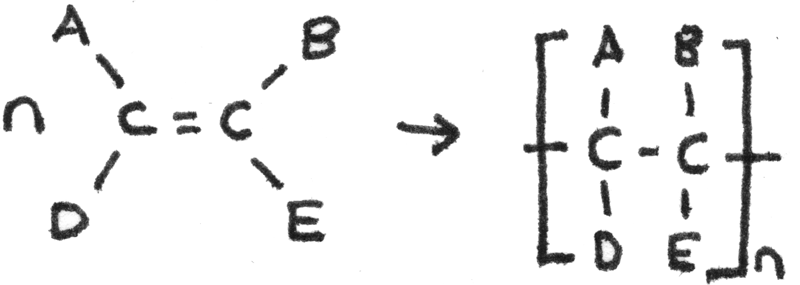
The table below shows a comparison between addition and condensation polymers.
| Product | Polyalkene | Polyester | Polyamide |
|---|---|---|---|
| Polymerisation | Addition | Condensation | Condensation |
| Functional groups | $C=C$ | $-COOH$ and $-OH$ | $-COOH$ and $-NH_{2}$ |
| Type of monomer | 1 | 1 or 2 | 1 or 2 |
Hydrolysis of Polyesters
Polyesters can be hydrolysed under acidic or basic conditions.
Base Hydrolysis
Polyesters are readily hydrolysed by hot aqueous alkalis, such as sodium hydroxide. Each ester linkage is hydrolysed to a carboxylate salt ($-COO^{-}Na^{+}$) and a hydroxyl group ($-OH$).
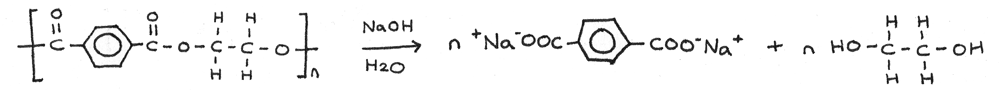
Acid Hydrolysis
In a much slower reaction, polyesters can be hydrolysed by hot aqueous acids, such as aqueous hydrochloric acid. Each ester linkage is hydrolysed to a carboxyl group ($-COOH$) and a hydroxyl group ($-OH$).
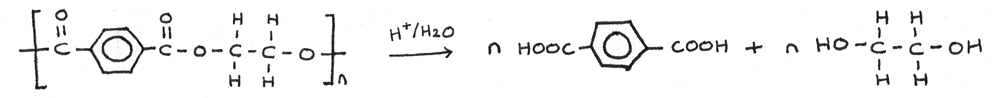
Hydrolysis of Polyamides
Polyamides can be hydrolysed under acidic or basic conditions.
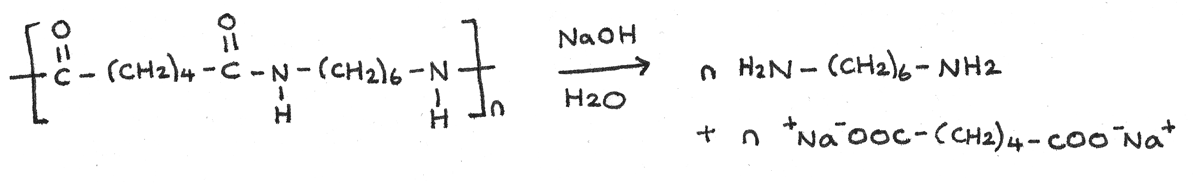
Base Hydrolysis
Polyamides can be hydrolysed with hot aqueous alkalis, such as sodium hydroxide. With base hydrolysis, the sodium salt of the dicarboxylic acid and a diamine are both formed.
Acid Hydrolysis
Polyamides can also be hydrolysed with hot aqueous acids, such as aqueous hydrochloric acid. With acid hydrolysis, a dicarboxylic acid and an ammonium salt of the diamine are produced.
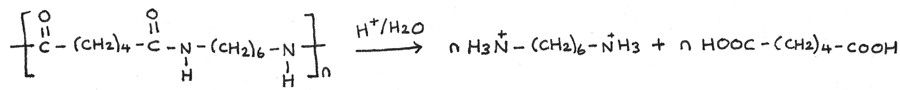
Degradable Polymers
Growing environmental concerns, customer demand and the increasing cost of fossil fuels had led to the development of renewable and sustainable polymers for consumer use. Such products include biodegradable carrier bags and packaging.
Biodegradable Polymers
Biodegradable polymers have bonds which will readily undergo hydrolysis allowing for easier breakdown of the polymer. Examples include:
- Polylactic acid made from lactic acid, derived from corn starch is often used in compostable bags and food packing.
- Polyglycolic acid which is made from glycolic acid isolated from sugar cane and unripe grapes. This polymer is mostly used for stitches in surgery.
Photodegradable Polymers
When exposed to sunlight, photodegradable polymers are designed to become weak and brittle. They are made by blending a polymer with light sensitive additives which catalyse the breakdown. Alternatively, carbonyl bonds ($C=O$) can be incorporated into the polymer which absorb energy and break down in prolonged sunlight. These polymers convert into waxy compounds when exposed to light before turning into carbon dioxide and water.