Fats and Oils
Fats and oils are important in the human body as they store energy, provide insulation and protect internal organs. They are also used in many commercial products such as margarine and processed foods.
Fatty Acids
Fatty acids are long chained carboxylic acids. They can either be unsaturated or saturated. A naturally occurring fatty acid usually contains an even number of carbon atoms due to the synthesis of the compound in nature.
The melting point is determined by the length of the hydrocarbon chain. The longer the chain, the higher the melting point. A fatty acid with a melting point below room temperature is known as a liquid oil. A fatty acid with a melting point above room temperature is known as a solid fat.
An example of a saturated fatty acid is palmitic acid ($C_{15}H_{31}COOH$).
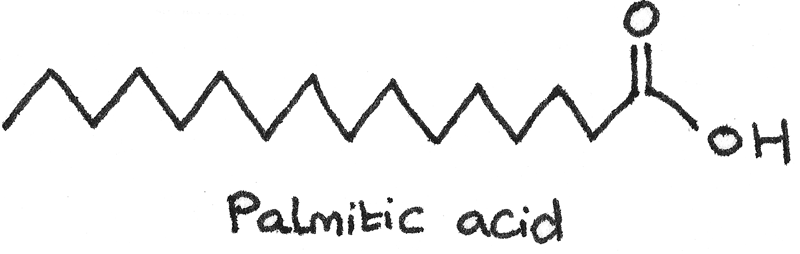
Unsaturated fats with one double bond in the hydrocarbon chain are called mono-unsaturated fats. As fatty acid chains can often be long, shorthand notation can be used. For example, octadec-9,12-dienoic acid can be represented as $18{:}2 (9,12)$.
- The first number indicates the number of carbon atoms.
- The second number indicates the number of $C=C$ double bonds.
- The numbers inside the brackets indicate the positions of the double bonds relative to the carbon atom in $-COOH$.
E/Z Isomerism
This type of stereoisomerism is present due to the restricted rotation around a $C=C$ double bond. Unsaturated fats can exist in two isomeric forms, either cis or trans.
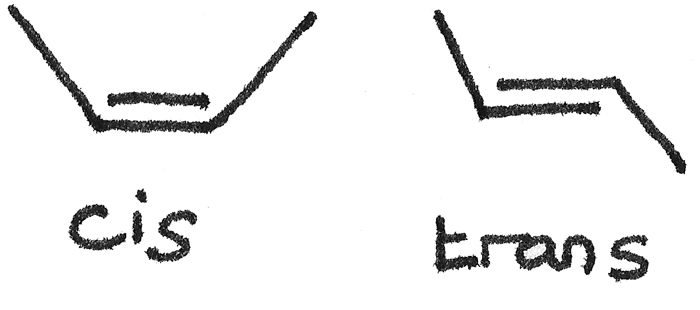
Unsaturated fatty acids make up the triglycerides found in vegetable and fish oils. Trans-fats are known to increase the risk of coronary heart disease. In nature, unsaturated fatty acids commonly exist in the cis form.
- Cis fatty acids cannot pack closely together and exist as liquids at room temperature.
- Trans fatty acids have a linear structure and can pack more closely together. This results in a higher melting point.
Triglycerides
A simple triglyceride is derived from one glycerol with three molecules of fatty acids. A triglyceride is a triester of the alcohol glycerol, which is a compound with the systematic name propane-1,2,3-triol.
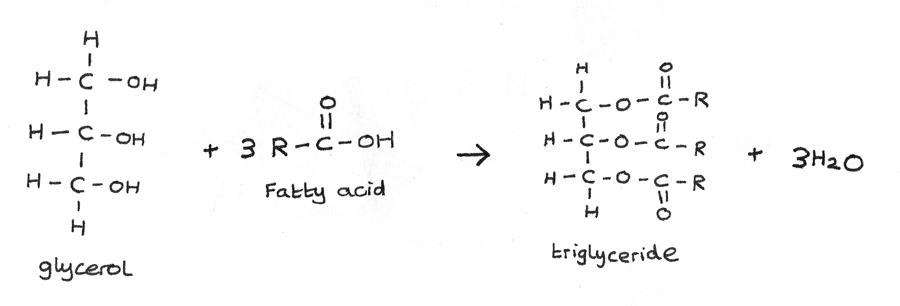
The fatty acid groups in triglycerides are joined to the glycerol through ester linkages. Triglycerides can either be saturated or unsaturated.
Fats In The Food Industry
Fats and oils are a part of a group of naturally occurring molecules called the lipids. Proteins that carry lipids in the body are called lipoproteins.
- High density lipoproteins (HDLs) transport cholesterol out of the blood and the body.
- Low density lipoproteins (LDLs) carry 65% of cholesterol in blood. LDLs can deposit lipids onto the artery walls, building up fatty deposits which restricts blood flow.
Trans fatty acids and saturated fats raise LDL levels and lower HDL levels. This increases the risk of heart disease.
Uses in Margarine
During margarine manufacture partial hydrogenation is often performed in order to make fats and oils more solid. This is a process where hydrogen atoms are added across double bonds. This is because the greater the number of $C=C$ double bonds, the lower the melting point. Hydrogenation can also unintentionally convert cis form double bonds to the less healthy trans form double bonds.
Biodiesel
The non renewable nature of fossil fuels has led to the development of biodiesels, which can be produced from waste cooking oil. Short chain fuels such as ethanol and methanol are renewable as they can be obtained from fermentation or plants. They also burn more efficiently and are cheap and easy to manufacture. Biodiesel can be produced through transesterification:
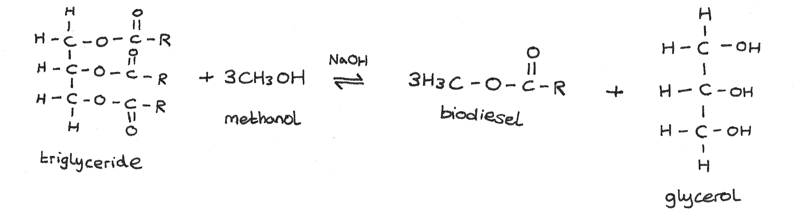
In this process, a triglyceride is reacted with methanol under alkaline conditions, forming biodiesel and glycerol.
Ethics of Biodiesel
An advantage of biodiesel is that it is carbon neutral, meaning it absorbs the same amount of carbon emissions as it releases. A disadvantage of biodiesel is that large areas of land must be consumed in order to grow the fuel crops.